Abstract
Background: Primary mediastinal B-cell lymphoma (PMBCL) is biologically related to classic nodular-sclerosis Hodgkin lymphoma. R-CHOP alone appears to be inadequate for many pts with retrospective studies indicating a benefit for consolidation XRT or use of intensive therapy regimens. End-of-therapy (EOT) FDG-PET is used to assess residual disease, but cannot accurately distinguish lymphoma from inflammation. We previously showed DA-EPOCH-R is effective in PMBCL, obviating the need for XRT in most pts, and showed that a positive EOT FDG-PET was typically not associated with treatment failure (Dunleavy et al. NEJM. 2013;368(15):1408-1416). We assessed the role of serial FDG-PET imaging to more accurately distinguish active disease from inflammation following DA-EPOCH-R for PMBCL.
Methods: Ninety-three untreated PMBCL pts received DA-EPOCH-R without XRT. EOT FDG-PET was performed in 83 (89%) pts within 1 week of completing treatment and retrospectively scored per Deauville criteria in a blinded manner. Twenty-five (44%) of 57 pts with a negative (Deauville 1-3) EOT scan underwent follow-up FDG-PET imaging, mostly due to Deauville 3, which is no longer considered to indicate a positive FDG-PET scan. Twenty-three (88%) of 26 pts with a positive (Deauville 4-5) EOT scan underwent serial FDG-PET imaging approximately every 6 weeks. Three Deauville 4 pts (all non-progressors) did not have follow-up scans per protocol. If progressive SUV changes were noted on follow-up PET and/or size increase on concurrent CT, biopsy was attempted to confirm disease prior to implementing salvage therapy.
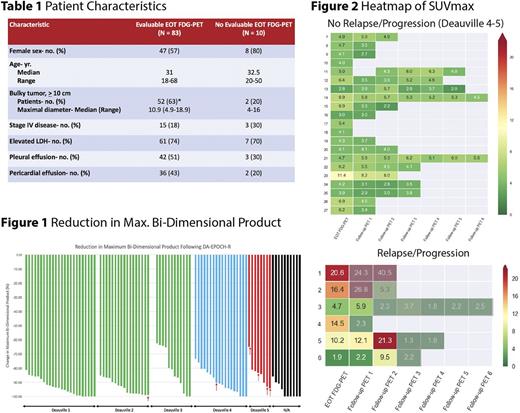
Results: Characteristics were similar between the 83 pts with and 10 pts without evaluable EOT FDG-PET scans, aside from greater bulky tumor >/= 10 cm in the former group (63% vs. 20%, P = 0.014) (Table 1). All 93 pts were evaluable for response by CT with an ORR of 99% (88% CR, 11% PR). All 89 pts with complete tumor measurements had a reduction in the bi-dimensional product of the largest mediastinal tumor mass by CT. There was no relationship between EOT tumor reduction and EOT FDG-PET Deauville score (Figure 1). Furthermore, there was no difference in tumor reduction between pts with (6) and without (83) treatment failure (red arrows); median reduction 92% (range, 65-99) vs. 93% (range, 62-100), respectively (Figure 1).
EOT FDG-PET scan was negative (Deauville 1-3) in 57 (69%) and positive (Deauville 4-5) in 26 (31%) pts. Disease progression occurred among 1/57 (2%) Deauville 1-3 pts, 1/18 (6%) Deauville 4 pts and 4/8 (50%) Deauville 5 pts. Two pts (both Deauville 5) died of progressive disease and 2 pts (Deauville 2 and 4, respectively) died without disease.
In the 83 pts with EOT FDG-PET scans, 113 follow-up scans in 48 pts were performed (median, 1; range, 0-6). Pts with a negative EOT FDG-PET scan underwent a median of 0 (range, 0-4) scans compared to a median of 2 (range, 0-6) scans in pts with a positive EOT scan. Among pts who did not relapse/progress, the SUVmax remained relatively stable over time in the 21 pts with positive (Figure 2, upper) and 56 pts with negative EOT FDG-PET scans (data not shown). Five of 6 pts with treatment failure had a progressive increase in SUVmax on serial imaging (Figure 2, lower). Four of these pts (#2, 3, 5, and 6) had normalization of SUVmax following intervention (indicated by gray diagonal bars) with 1 pt (#1) having continued progression despite multiple salvage therapies. One patient (#4) with an elevated SUVmax at EOT had normalization of uptake following XRT.
Conclusions: The degree of EOT tumor reduction by CT scan was unrelated to FDG-PET Deauville score or risk of disease recurrence. Negative EOT FDG-PET scans are highly predictive of cure following DA-EPOCH-R and further FDG-PET scanning should not be performed. Positive EOT FDG-PET scans were only indicative of residual disease in a minority of pts and should not be used alone to identify pts with residual disease. However, serial scans can identify pts with residual disease who require treatment. In conclusion, serial imaging should be performed in pts with positive EOT FDG-PET scans to accurately identify pts with disease.
Advani: Merck: Research Funding; Agensys: Research Funding; Pharmacyclics: Research Funding; Nanostring: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Regeneron: Research Funding; Juno Therapeutics: Consultancy; Sutro: Consultancy; Infinity: Research Funding; FortySeven: Research Funding; Janssen: Research Funding; Pharmacyclics: Consultancy; Bayer Healthcare Pharmaceuticals: Research Funding; Cell Medica: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding; Kura: Research Funding; Gilead: Consultancy; Genentech: Research Funding; Spectrum: Consultancy; Millennium: Research Funding. Miljkovic: Pfizer Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal